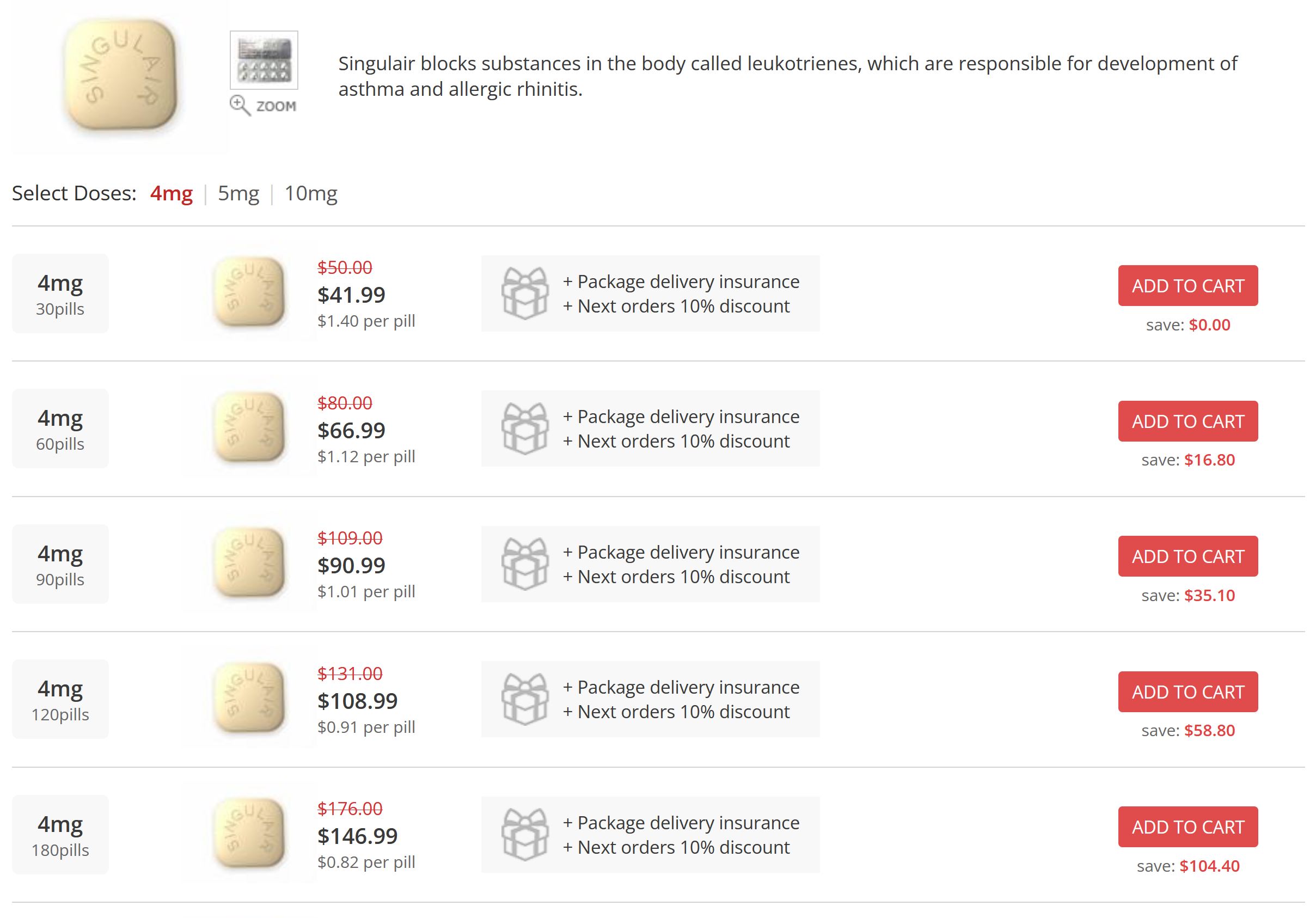
How can I buy Singulair/Montelukast online?
Overview of Singulair
Singulair, known scientifically as montelukast, is a medication widely used in the management of asthma and allergic rhinitis. It falls under the category of leukotriene receptor antagonists, a class of drugs that help to control allergic reactions and asthma symptoms by blocking the action of leukotrienes, which are substances in the body that can cause asthma and allergy symptoms.
Uses of Singulair
Asthma: Singulair is prescribed to prevent asthma attacks in adults and children as young as 12 months old. It is not a rescue medicine for asthma attacks but rather is used to prevent them over the long term and to decrease the frequency and severity of episodes.
Allergic Rhinitis: It's also effective in treating symptoms of allergic rhinitis, often known as hay fever, such as sneezing, stuffy nose, runny nose, and itching of the nose.
Exercise-Induced Bronchoconstriction: Additionally, Singulair is used to prevent exercise-induced bronchoconstriction (EIB), which is narrowing of the airways that happens with exercise, in patients 6 years of age and older.
How It Works
Montelukast works by blocking leukotrienes, which are chemicals in the body that react to different allergens like pollen, dust mites, or pet dander. By inhibiting these chemicals, Singulair helps to reduce inflammation, decrease mucus secretion, and prevent airway edema, thereby making breathing easier and preventing asthma episodes and allergy symptoms.
Administration
Singulair is typically administered once daily in the evening, which can be helpful in controlling asthma or allergy symptoms at night. The tablets, chewable tablets, and granules forms are available to accommodate various ages and preferences.
Side Effects
While Singulair is generally well-tolerated, it can cause side effects in some individuals. Common side effects include stomach pain, heartburn, upset stomach, nausea, diarrhea, and headache. It’s important to discuss with a healthcare provider if any severe side effects occur, such as mood changes, signs of liver damage, or a severe allergic reaction.
Adverse reactions - Frequency
- Infections and infestations Upper respiratory tract infections - Very common
- Blood and lymphatic system disorders Tendency to increase bleeding - Rare
- Thrombocytopenia Very rare
- Immune system disorders Hypersensitivity reactions, including anaphylaxis - Uncommon
- Eosinophilic infiltration of the liver - Very rare
- Psychiatric disorders Sleep disturbances, including nightmares, insomnia, somnambulism, anxiety, agitation, including aggressive behavior or hostility, depression, psychomotor hyperactivity - Uncommon
- Attention impairment, memory impairment - Rare
- Hallucinations, disorientation, suicidal thoughts and behavior, obsessive-compulsive disorder, dysphemia - Very rare
- Nervous system disorders Dizziness, lethargy, paresthesia/hypoesthesia, seizures - Uncommon
- Cardiac disorders Palpitations - Rare
- Respiratory, thoracic and mediastinal disorders Epistaxis - Uncommon
- Churg-Strauss syndrome pulmonary eosinophilia - Very rare
- Gastrointestinal disorders Diarrhea, nausea, vomiting - Common
- Dry mouth, dyspepsia - Uncommon
- Hepatobiliary disorders Increased plasma transaminases - Common
- Hepatitis - Very rare
- Skin and subcutaneous tissue disorders Rash - Common
- Hematoma, urticaria, pruritus - Uncommon
- Angioedema - Rare
- Erythema nodosum, erythema multiforme - Very rare
- Musculoskeletal and connective tissue disorders Arthralgia, myalgia, including muscle convulsions - Uncommon
- Renal and urinary tract disorders Enuresis in children - Uncommon
- General disorders and adverse effects due to taking the drug Pyrexia - Common
- Asthenia/fatigue, malaise, oedema - Uncommon
How can I buy Singulair (Montelukast) online?
To order the drug over the counter (otc), follow the advertising link of the Canadian online pharmacy of generics. There you can buy a generic Singulair inexpensively. A prescription from a doctor is not required to buy generics.
We offer the best price and high quality of medicines, and also guarantee anonymity. Mail delivery of the order to any place. For repeat purchases, a discount of up to 20% is given.
Considerations and Precautions
It's crucial to use Singulair under the guidance of a healthcare provider. It shouldn’t be used as a substitute for inhaled or oral corticosteroids. Also, it's not intended for the immediate relief of acute asthma attacks. Patients should always have a fast-acting bronchodilator medication on hand to treat asthma attacks.
Placebo, Comparisons and Studies
In an 8-week study in children aged 6 to 14 years, montelukast 5 mg once daily compared with placebo significantly improved respiratory function (change from baseline in FEV1: 8.71% vs. 4.16%, change in morning PEF: 27.9 L/min vs. 17.8 L/min) and reduced the frequency of on-demand β-agonist use (change from baseline by -11.7% vs. +8.2%).
In a 12-week, placebo-controlled study in children 2 to 5 years of age, montelukast 4 mg once daily improved asthma control compared with placebo, regardless of concomitant controller therapy (inhaled/nebulized corticosteroids, inhaled/nebulized sodium cromoglycate). Sixty percent of patients did not receive other controller therapy. Singulair improved daytime symptoms (including cough, wheeze, shortness of breath, and activity limitation) and nighttime symptoms compared with placebo. Singulair also reduced the use of on-needed beta-agonists and rescue corticosteroids for worsening asthma compared with placebo. Patients taking montelukast experienced more asthma-free days than those taking placebo.
The therapeutic effect was achieved after the first dose.
In a 12-month, placebo-controlled study in children aged 2 to 5 years with mild asthma and episodic exacerbations, montelukast 4 mg once daily significantly (p≤0.001) reduced the annualized rate of asthma exacerbations compared with placebo (1.60 vs. 2.34 exacerbations, respectively) [exacerbations defined as ≥3 consecutive days of daytime symptoms requiring β-agonists or corticosteroids (oral or inhaled), or hospitalization for asthma treatment].
The proportion of reduction in the annualized rate of exacerbations was 31.9% (95% CI 16.9, 44.1). In a placebo-controlled study in children 6 months to 5 years of age with intermittent (but not persistent) asthma, montelukast treatment was continued for 12 months at a regimen of 4 mg once daily or in 12-day courses, with each course initiated when an episode of intermittent symptoms occurred.
There was no significant difference between patients treated with montelukast 4 mg and those treated with placebo in the number of asthma episodes progressing to an asthma attack (defined as an asthma episode requiring an unscheduled physician, emergency room, or clinic visit; or treatment with oral, IV, or IM corticosteroids).
In a 12-month study comparing the efficacy of montelukast and inhaled fluticasone for asthma control in children aged 6 to 14 years with mild persistent asthma, montelukast was noninferior to fluticasone in increasing the percentage of days free from the use of rapid-relief medications (the primary endpoint).
On average, over the 12-month treatment period, the percentage of days free from the use of rescue medications increased from 61.6 to 84.0 in the montelukast group and from 60.9 to 86.7 in the fluticasone group.
The difference in the percentage of mean square (LS) increase in days free from the use of rapid-relief medications between groups was statistically significant (–2.8; 95% CI –4.7, –0.9), but within the prespecified clinical noninferiority range.
Montelukast and fluticasone also improved asthma control on secondary endpoints assessed over the 12-month treatment period.
FEV1 increased from 1.83 to 2.09 L in the montelukast group and from 1.85 to 2.14 L in the fluticasone group.
- The between-group LS difference in FEV1 increase was -0.02 L (95% CI -0.06, 0.02).
- The mean percentage increase from baseline in predicted FEV1 was 0.6% in the montelukast group and 2.7% in the fluticasone group.
- The LS difference was significant: -2.2% (95% CI -3.6, -0.7).
- The percentage of days with β-agonist use decreased from 38.0 to 15.4% in the montelukast group and from 38.5 to 12.8% in the fluticasone group.
- The between-group difference in the LS value for the percentage of days with β-agonist use was significant, 2.7 (95% CI 0.9; 4.5).
- The percentage of patients with asthma attacks (an asthma attack is defined as a period of worsening asthma requiring treatment with oral steroids, an unscheduled visit to the doctor, emergency care, or hospitalization) was 32.2% in the montelukast group and 25.6% in the fluticasone group; the odds ratio (95% CI) was significant, 1.38 (1.04; 1.84).
- The proportion of patients using systemic (mainly oral) GCS during the study period was 17.8% in the montelukast group and 10.5% in the fluticasone group.
- The intergroup difference in the LS indicator was significant: 7.3% (95% CI 2.9; 11.7).
Conclusion
Singulair is a significant advancement in the management of asthma and allergic rhinitis, offering convenience with once-daily dosing and the potential for substantial relief of symptoms. However, like any medication, it is vital to use it under the direction of a healthcare professional to ensure it is appropriate for your condition and to monitor for any possible side effects.